TSE PhenoMaster Automated Home Cage Phenotyping

About this resource
The State Council of Higher Education for Virginia and Virginia College Building Authority (VCBA) jointly administered Higher Education Equipment Trust Fund (HEETF) graciously supported the acquisition of the TSE PhenoMaster Mouse Phenotyping System at VCU. The instrument is managed by Francesco S. Celi, MD, MHSc, within the Department of Internal Medicine; Division of Endocrinology, Diabetes, and Metabolism and is housed in Sanger Hall Room 7-034.
Those interested in using this resource should contact Bin Ni, PhD.
The PhenoMaster System (TSE Systems) allows long-term measurement of multiple parameters within the mouse Home Cage. The system accommodates up to 12 Home Cages, which are housed in a climate controlled chamber allowing manipulation of temperature (4-35°C), humidity, and the light/dark cycle.
Indirect Calorimetry Principle
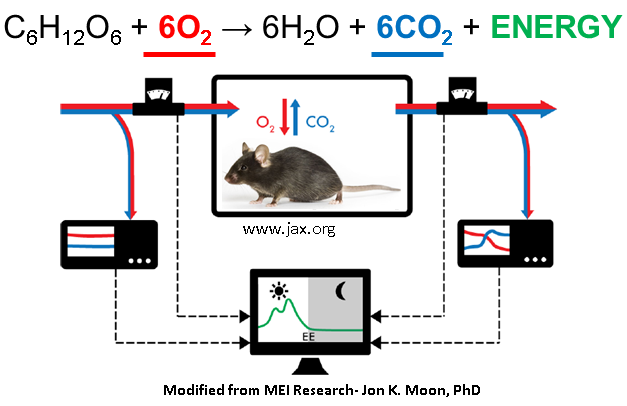
Indirect calorimetry measures oxygen consumption ( red ) and carbon dioxide production (blue) to provide an indirect assessment of energy production (green).
Home Cage Measurements:

- Indirect calorimetry (Pull) with the following derived values
- Energy Expenditure (kcal/hr)
- VO2 consumed
- VCO2 produced
- Respiratory Exchange Ratio (VCO2 produced/VO2 consumed)
- RER ≥ 1 ; Carbohydrates as predominate fuel source
- RER = 0.85 ; Mixed Fat and Carbohydrate as fuel source
- RER = 0.7 ; Fat as predominate fuel source
- Spontaneous body weight measurement
- Food intake
- Water intake
- Activity(X, Y, Z: IR beam-break)
- Spacial activity analysis and charting
- Total beam breaks and area
- Centered beam breaks and area
- Peripheral beam breaks and area
- Total distance
- Instantaneous and average speed
- Sleep Analysis
- Voluntary wheel running (programmable Enable/Disable Function)
- Running Wheel Turns
- Run Time
- Run Attempts
- Maximum Running Speed
- Average Running Speed
- Maximum Run Distance
- APC uninterrupted power supply with 2,200 VA and 1,920-watt capacity for added experimental and animal welfare protection
Metabolic PhenoCages

An additional 12 PhenoCages are available to measure the following parameters, providing important information about the animal’s energy balance.
- Indirect calorimetry (Pull)
- Food intake
- Water intake
- Separation and collection of urine
- Separation and collection of feces
Stellar Telemetry Units

- 6 wireless, implantable transmitters capable of measuring core body temperature, brown adipose tissue temperature, and activity are available for study use.
- The Stellar Telemetry system is expandable to include transmitters for heart rate, ECG and other important physiological measurements.
FLIR T420 Infrared Imaging Camera
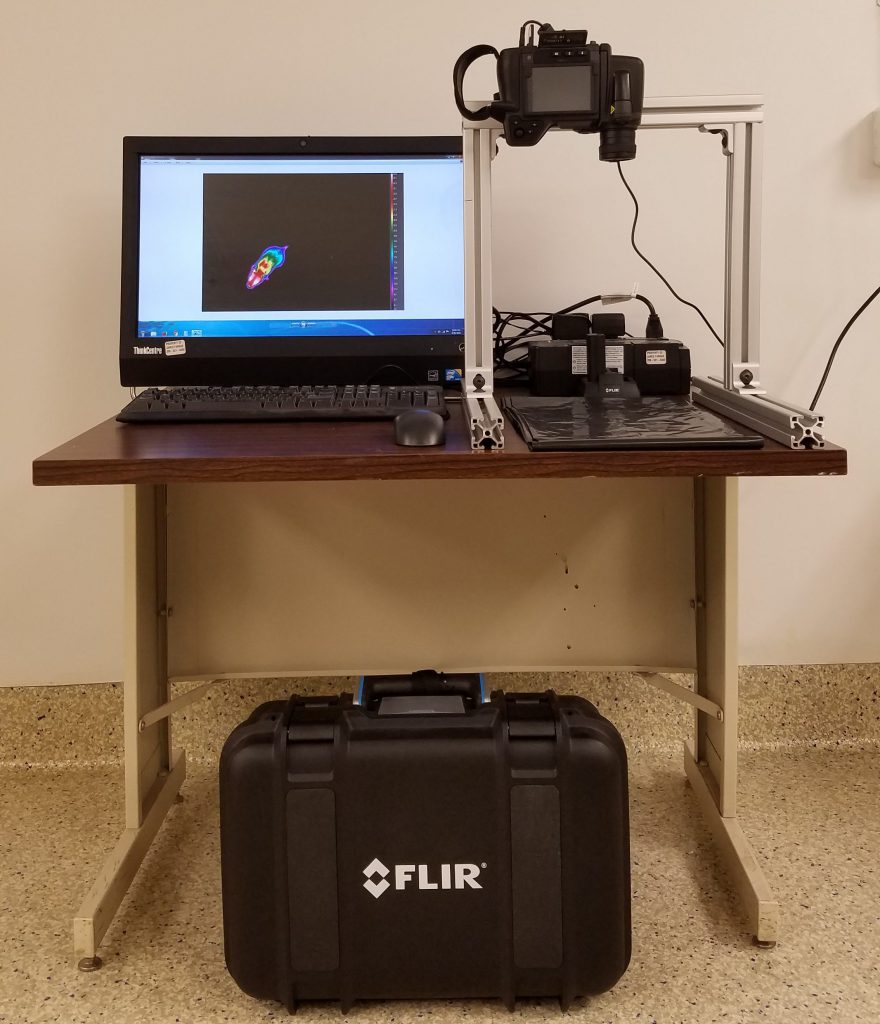
The infrared imaging system enables continuous real-time monitoring of animal well-being during experimental and surgical manipulations.
- High Thermal Sensitivity (N.E.T.D): <0.045°C @ 30°C
- Temperature Accuracy: ±2%
- Focal Plane Array detector with 320 x 240 pixels infrared resolution (76,800 pixels)
- Reflected ambient temperature and emissivity correction
- Multi-Spectral Dynamic Imaging (MSX): Overlays visible light images with IR images in real-time for extraordinary thermal detail
- 25° and 45° lenses
Powers Scientific Rodent Incubator

- Model RIS33SD
- 12-24 cage capacity
- Programmable temperature control and light/dark cycle
- APC uninterrupted power supply with 2,200 VA and 1,920-watt capacity for added experimental and animal welfare protection
Data Analysis Resources

CalR: A Web Application for Indirect Calorimetry Analysis
An excellent resource for the analysis of indirect calorimetry data developed by the laboratory of Alexander Banks, PhD at the Beth Israel Deaconess Medical Center.
CalR: A Web-Based Analysis Tool for Indirect Calorimetry Experiments. Mina AI, LeClair RA, LeClair KB, Cohen DE, Lantier L, Banks AS. Cell Metab. 2018 Oct 2;28(4):656-666.e1.
Article DOI: https://doi.org/10.1016/j.cmet.2018.06.019
From the abstract of the manuscript:
We report a web-based tool for analysis of experiments using indirect calorimetry to measure physiological energy balance. CalR simplifies the process to import raw data files, generate plots, and determine the most appropriate statistical tests for interpretation. Analysis using the generalized linear model (which includes ANOVA and ANCOVA) allows for flexibility in interpreting diverse experimental designs, including those of obesity and thermogenesis. Users also may produce standardized output files for an experiment that can be shared and subsequently re-evaluated using CalR. This framework will provide the transparency necessary to enhance consistency, rigor, and reproducibility. The CalR analysis software will greatly increase the speed and efficiency with which metabolic experiments can be organized, analyzed per accepted norms, and reproduced and will likely become a standard tool for the field.

National Institutes of Health sponsored resource National Mouse Metabolic Phenotyping Centers (MMPC) Energy Expenditure Analysis Page
Useful description and case studies detailing the use of multiple linear regression analysis to asses the impact of covariates (eg Mass) on energy expenditure measurements.
https://www.mmpc.org/shared/regression.aspx
INITIAL SETUP OF THE PHENOMASTER IN SANGER HALL
Almost empty room ready for the rest of the PhenoMaster to arrive 06/27/2018

Note: JS Farrar added the window treatments (blacked out lining) to the windows in this room to make the space VCU IACUC compatible.
Cargo shipping label

Removing the climate chamber from its crate

Everything ready to be unpacked 09/17/2018


Note: Not shown is the amount of work it took to get these into the room. Many thanks to Joe Lownik for his assistance!
PhenoMaster in the dark 09/21/2018